- Medical Device Training
- >
- MDR Training 2022
MDR Training 2022
SKU:
$400.00
$250.00
$250.00
Unavailable
per item
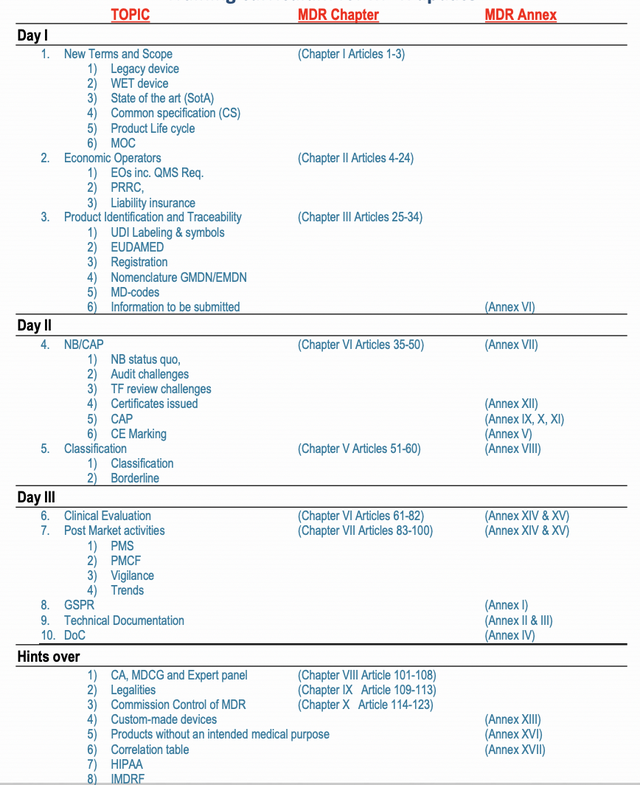
Breakthrough Medical Device Regulation
with a clear emphasis toward [State of the Art], [Well established Technology].
The training will allow you to understand all MDR definition
The training will break through all articles without exemption.
The training will correlate MDR articles with MDCG guides, Hands-on Technical file structure, and GSPR.
The training will help you to integrate MDR Article 10 into your QMS ISO 13485
The Training will allow you to augment the role of Economic operators EOs into the current QMS.
The training will shed light on potential Major common non-conformance, and the reason for rejection of Technical files or possible delays.